Description
Generic Name
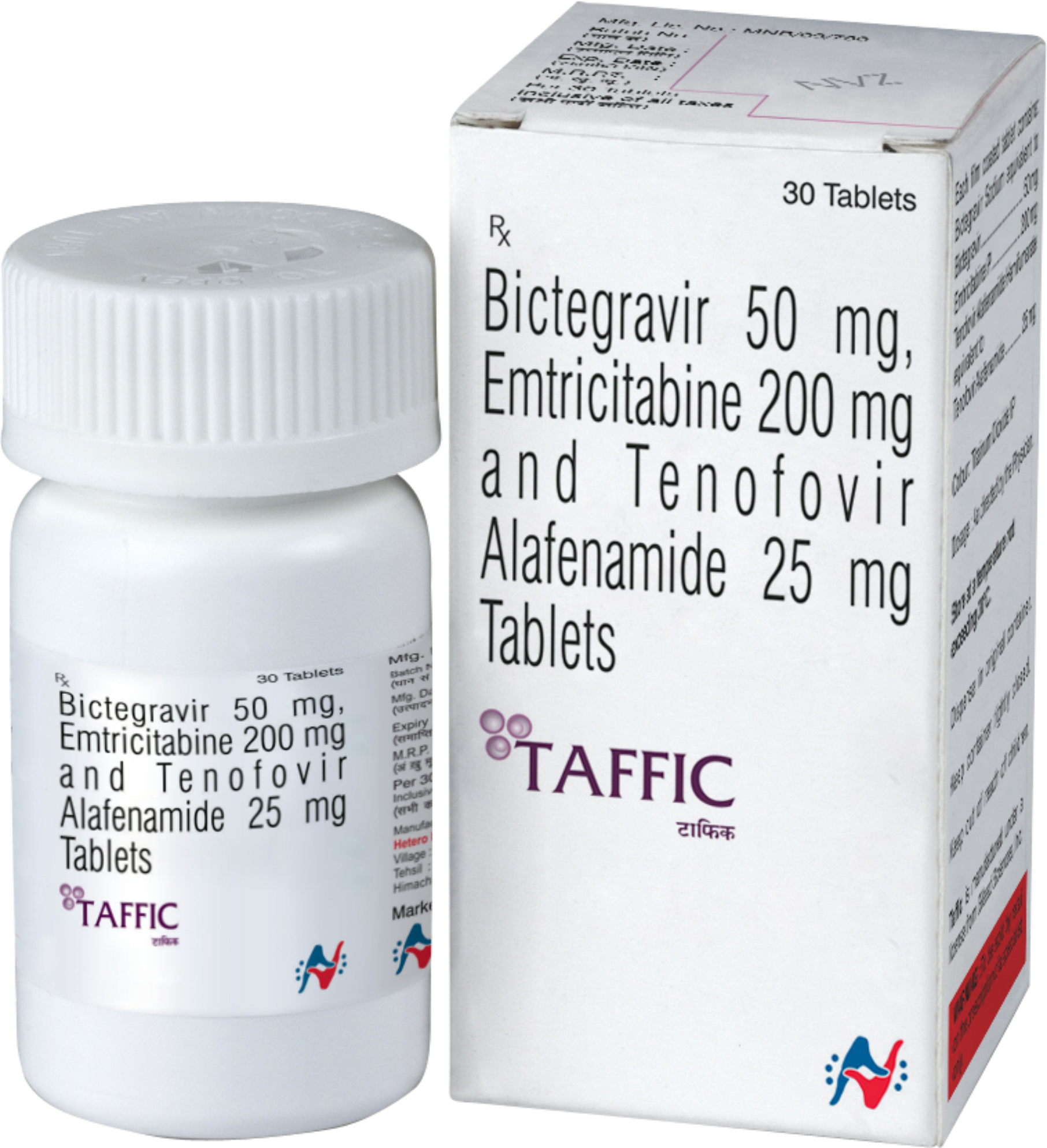
Taffic Tablet contains Bictegravir, Emtricitabine, and Tenofovir Alafenamide.
Composition:
Each Taffic Tablet comprises:
- Bictegravir (50 mg)
- Emtricitabine (200 mg)
- Tenofovir Alafenamide (25 mg)
Indication:
Taffic is used for the treatment of HIV-1 infection in adults and adolescents. It is prescribed as a single-tablet regimen for patients who have no known resistance to integrase inhibitors, nucleoside reverse transcriptase inhibitors (NRTIs), or tenofovir.
Storage Instructions:
- Store at room temperature (25°C) in a dry place.
- Keep away from direct sunlight and moisture.
- Keep out of reach of children.
Uses of the Medicine:
- Helps manage and treat HIV-1 infection.
- Reduces viral load and increases CD4 cell count.
- Helps prevent HIV progression to AIDS.
Benefits of the Medicine:
- Convenient single-tablet regimen for HIV treatment.
- Effectively suppresses HIV when taken regularly.
- Strengthens the immune system by reducing HIV in the blood.
- Lowers the risk of HIV transmission.
Side Effects:
Common side effects include:
- Nausea
- Diarrhea
- Fatigue
- Headache
- Insomnia
- Dizziness
Serious Side Effects Include:
- Liver toxicity (yellowing of skin/eyes, dark urine)
- Lactic acidosis (muscle pain, weakness, breathing difficulty)
- Severe allergic reactions (rash, swelling, difficulty breathing)
- Kidney problems (increased thirst, changes in urination)
How to Use the Medicine:
- Take one tablet daily at the same time each day.
- Can be taken with or without food.
- Swallow whole with water; do not chew or crush.
- Do not miss doses to maintain viral suppression.
How the Medicine Works:
- Taffic is a fixed-dose combination antiretroviral that contains:
- Bictegravir: An integrase strand transfer inhibitor (INSTI) that blocks viral replication.
- Emtricitabine & Tenofovir Alafenamide: Nucleoside reverse transcriptase inhibitors (NRTIs) that prevent HIV from copying itself.
- Together, these components help reduce HIV viral load and slow disease progression.
What If You Forget to Take the Medicine?
- Take the missed dose as soon as you remember.
- If it’s almost time for the next dose, skip the missed dose.
- Do not double the dose to compensate.
Quick Tips:
- Take Taffic daily to ensure effective HIV suppression.
- Regular HIV viral load and CD4 tests are necessary to monitor treatment effectiveness.
- Do not share your medication with others.
- Inform your doctor if you have a history of liver or kidney disease.
Safety Advice

Alcohol
Caution is advised when consuming alcohol with Taffic Tablet. Please consult your doctor for guidance.
Pregnancy
Taffic Tablet is unsafe to use during pregnancy due to potential harm to the developing baby. It may only be prescribed in life-threatening situations where benefits outweigh the risks. Please consult your doctor.
Breastfeeding
There is insufficient information on the use of Taffic Tablet while breastfeeding. Please consult your doctor before use.
Driving
It is uncertain whether Taffic Tablet affects your ability to drive. Avoid driving if you experience drowsiness, dizziness, or reduced alertness.
Kidney
Limited data is available on the safety of Taffic Tablet in patients with kidney disease. Dose adjustments may be necessary. Please consult your doctor.
Liver
Taffic Tablet should be used with caution in patients with liver disease. Dose adjustments may be required based on the severity of the condition. Please consult your doctor.
FAQs:
Q: Can Taffic cure HIV?
A: No, Taffic does not cure HIV but helps manage and control the infection.
Q: Can I stop taking Taffic if I feel better?
A: No, stopping Taffic without consulting your doctor may cause the virus to become resistant.
Q: Does Taffic interact with other medications?
A: Yes, inform your doctor about all medications you are taking to avoid interactions
Related Lab Tests:
- HIV viral load test
- CD4 count
- Liver function test
- Kidney function test
Other Resources























Reviews
There are no reviews yet.